Antioxidant
Contents
Antioxidant
From Wikipedia, the free encyclopedia
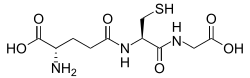
Structure of the antioxidant glutathione.
Antioxidants are compounds that inhibit oxidation. Oxidation is a chemical reaction that can produce free radicals, thereby leading to chain reactions that may damage the cells of organisms. Antioxidants such as thiols or ascorbic acid (vitamin C) terminate these chain reactions. To balance the oxidative state, plants and animals maintain complex systems of overlapping antioxidants, such as glutathione and enzymes (e.g., catalase and superoxide dismutase), produced internally, or the dietary antioxidants vitamin C, and vitamin E.
The term "antioxidant" is mostly used for two entirely different groups of substances: industrial chemicals that are added to products to prevent oxidation, and naturally occurring compounds that are present in foods and tissue. The former, industrial antioxidants, have diverse uses: acting as preservatives in food and cosmetics, and being oxidation-inhibitors in fuels.[1]
Importantly, antioxidant dietary supplements have not been shown to improve health in humans, or to be effective at preventing disease.[2] Supplements of beta-carotene, vitamin A, and vitamin E have no positive effect on mortality rate[3][4] or cancer risk.[5][6] Additionally, supplementation with selenium or vitamin E do not reduce the risk of cardiovascular disease.[7][8]
Examples of bioactive antioxidant compounds
Antioxidants are classified into two broad divisions, depending on whether they are soluble in water (hydrophilic) or in lipids (lipophilic). In general, water-soluble antioxidants react with oxidants in the cell cytosol and the blood plasma, while lipid-soluble antioxidants protect cell membranes from lipid peroxidation.[49] These compounds may be synthesized in the body or obtained from the diet.[50] The different antioxidants are present at a wide range of concentrations in body fluids and tissues, with some such as glutathione or ubiquinone mostly present within cells, while others such as uric acid are more evenly distributed (see table below). Some antioxidants are only found in a few organisms and these compounds can be important in pathogens and can be virulence factors.[68]
The relative importance and interactions between these different antioxidants is a very complex question, with the various antioxidant compounds and antioxidant enzyme systems having synergistic and interdependent effects on one another.[69][70] The action of one antioxidant may therefore depend on the proper function of other members of the antioxidant system.[50]The amount of protection provided by any one antioxidant will also depend on its concentration, its reactivity towards the particular reactive oxygen species being considered, and the status of the antioxidants with which it interacts.[50]
Some compounds contribute to antioxidant defense by chelating transition metals and preventing them from catalyzing the production of free radicals in the cell. Particularly important is the ability to sequester iron, which is the function of iron-binding proteins such as transferrin and ferritin.[62] Selenium and zinc are commonly referred to as antioxidant nutrients, but these chemical elements have no antioxidant action themselves and are instead required for the activity of some antioxidant enzymes, as is discussed below.
| Antioxidant | Solubility | Concentration in human serum (μM) | Concentration in liver tissue (μmol/kg) |
|---|---|---|---|
| Ascorbic acid (vitamin C) | Water | 50–60[71] | 260 (human)[72] |
| Glutathione | Water | 4[73] | 6,400 (human)[72] |
| Lipoic acid | Water | 0.1–0.7[74] | 4–5 (rat)[75] |
| Uric acid | Water | 200–400[76] | 1,600 (human)[72] |
| Carotenes | Lipid | β-carotene: 0.5–1[77] | 5 (human, total carotenoids)[79] |
| α-Tocopherol (vitamin E) | Lipid | 10–40[78] | 50 (human)[72] |
| Ubiquinol (coenzyme Q) | Lipid | 5[80] | 200 (human)[81] |
Uric acid
Uric acid is by far the highest concentration antioxidant in human blood. Uric acid (UA) is an antioxidant oxypurine produced from xanthine by the enzyme xanthine oxidase, and is an intermediate product of purine metabolism.[82] In almost all land animals, urate oxidase further catalyzes the oxidation of uric acid to allantoin,[83] but in humans and most higher primates, the urate oxidase gene is nonfunctional, so that UA is not further broken down.[83][84] The evolutionary reasons for this loss of urate conversion to allantoin remain the topic of active speculation.[85][86] The antioxidant effects of uric acid have led researchers to suggest this mutation was beneficial to early primates and humans.[86][87] Studies of high altitude acclimatization support the hypothesis that urate acts as an antioxidant by mitigating the oxidative stress caused by high-altitude hypoxia.[88]
Uric acid has the highest concentration of any blood antioxidant[76] and provides over half of the total antioxidant capacity of human serum.[89] Uric acid's antioxidant activities are also complex, given that it does not react with some oxidants, such as superoxide, but does act against peroxynitrite,[90] peroxides, and hypochlorous acid.[82] Concerns over elevated UA's contribution to gout must be considered as one of many risk factors.[91] By itself, UA-related risk of gout at high levels (415–530 μmol/L) is only 0.5% per year with an increase to 4.5% per year at UA supersaturation levels (535+ μmol/L).[92] Many of these aforementioned studies determined UA's antioxidant actions within normal physiological levels,[88][90] and some found antioxidant activity at levels as high as 285 μmol/L.[93]
Vitamin C
Ascorbic acid or "vitamin C" is a monosaccharide oxidation-reduction (redox) catalyst found in both animals and plants. As one of the enzymes needed to make ascorbic acid has been lost by mutation during primate evolution, humans must obtain it from the diet; it is therefore a vitamin.[94] Most other animals are able to produce this compound in their bodies and do not require it in their diets.[95] Ascorbic acid is required for the conversion of the procollagen to collagen by oxidizing proline residues to hydroxyproline. In other cells, it is maintained in its reduced form by reaction with glutathione, which can be catalysed by protein disulfide isomerase and glutaredoxins.[96][97] Ascorbic acid is a redox catalyst which can reduce, and thereby neutralize, reactive oxygen species such as hydrogen peroxide.[98] In addition to its direct antioxidant effects, ascorbic acid is also a substrate for the redox enzyme ascorbate peroxidase, a function that is particularly important in stress resistance in plants.[99] Ascorbic acid is present at high levels in all parts of plants and can reach concentrations of 20 millimolar in chloroplasts.[100]
Glutathione
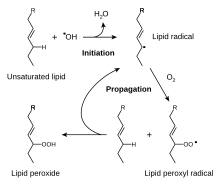
The free radical mechanism of lipid peroxidation.
Glutathione is a cysteine-containing peptide found in most forms of aerobic life.[101] It is not required in the diet and is instead synthesized in cells from its constituent amino acids.[102] Glutathione has antioxidant properties since the thiol group in its cysteine moiety is a reducing agent and can be reversibly oxidized and reduced. In cells, glutathione is maintained in the reduced form by the enzyme glutathione reductase and in turn reduces other metabolites and enzyme systems, such as ascorbate in the glutathione-ascorbate cycle, glutathione peroxidases and glutaredoxins, as well as reacting directly with oxidants.[96] Due to its high concentration and its central role in maintaining the cell's redox state, glutathione is one of the most important cellular antioxidants.[101] In some organisms glutathione is replaced by other thiols, such as by mycothiol in the Actinomycetes, bacillithiol in some Gram-positive bacteria,[103][104] or by trypanothione in the Kinetoplastids.[105][106]
Vitamin E
Vitamin E is the collective name for a set of eight related tocopherols and tocotrienols, which are fat-soluble vitamins with antioxidant properties.[107][108] Of these, α-tocopherol has been most studied as it has the highest bioavailability, with the body preferentially absorbing and metabolising this form.[109]
It has been claimed that the α-tocopherol form is the most important lipid-soluble antioxidant, and that it protects membranes from oxidation by reacting with lipid radicals produced in the lipid peroxidation chain reaction.[107][110] This removes the free radical intermediates and prevents the propagation reaction from continuing. This reaction produces oxidised α-tocopheroxyl radicals that can be recycled back to the active reduced form through reduction by other antioxidants, such as ascorbate, retinol or ubiquinol.[111] This is in line with findings showing that α-tocopherol, but not water-soluble antioxidants, efficiently protects glutathione peroxidase 4 (GPX4)-deficient cells from cell death.[112] GPx4 is the only known enzyme that efficiently reduces lipid-hydroperoxides within biological membranes.
However, the roles and importance of the various forms of vitamin E are presently unclear,[113][114] and it has even been suggested that the most important function of α-tocopherol is as a signaling molecule, with this molecule having no significant role in antioxidant metabolism.[115][116] The functions of the other forms of vitamin E are even less well understood, although γ-tocopherol is a nucleophile that may react with electrophilic mutagens,[109] and tocotrienols may be important in protecting neurons from damage.[117]
Oxidative challenge in biology
Further information: Oxidative stress
The structure of the antioxidant vitamin ascorbic acid (vitamin C).
A paradox in metabolism is that, while the vast majority of complex life on Earth requires oxygen for its existence, oxygen is a highly reactive molecule that damages living organisms by producing reactive oxygen species.[48] Consequently, organisms contain a complex network of antioxidant metabolites and enzymes that work together to prevent oxidative damage to cellular components such as DNA, proteins and lipids.[49][50] In general, antioxidant systems either prevent these reactive species from being formed, or remove them before they can damage vital components of the cell.[48][49] However, reactive oxygen species also have useful cellular functions, such as redox signaling. Thus, the function of antioxidant systems is not to remove oxidants entirely, but instead to keep them at an optimum level.[51]
The reactive oxygen species produced in cells include hydrogen peroxide (H2O2), hypochlorous acid (HClO), and free radicals such as the hydroxyl radical (·OH) and the superoxide anion (O2−).[52] The hydroxyl radical is particularly unstable and will react rapidly and non-specifically with most biological molecules. This species is produced from hydrogen peroxide in metal-catalyzed redox reactions such as the Fenton reaction.[53] These oxidants can damage cells by starting chemical chain reactions such as lipid peroxidation, or by oxidizing DNA or proteins.[49] Damage to DNA can cause mutations and possibly cancer, if not reversed by DNA repair mechanisms,[54][55] while damage to proteins causes enzyme inhibition, denaturation and protein degradation.[56]
The use of oxygen as part of the process for generating metabolic energy produces reactive oxygen species.[57] In this process, the superoxide anion is produced as a by-product of several steps in the electron transport chain.[58] Particularly important is the reduction of coenzyme Q in complex III, since a highly reactive free radical is formed as an intermediate (Q·−). This unstable intermediate can lead to electron "leakage", when electrons jump directly to oxygen and form the superoxide anion, instead of moving through the normal series of well-controlled reactions of the electron transport chain.[59] Peroxide is also produced from the oxidation of reduced flavoproteins, such as complex I.[60]However, although these enzymes can produce oxidants, the relative importance of the electron transfer chain to other processes that generate peroxide is unclear.[61][62] In plants, algae, and cyanobacteria, reactive oxygen species are also produced during photosynthesis,[63] particularly under conditions of high light intensity.[64] This effect is partly offset by the involvement of carotenoids in photoinhibition, and in algae and cyanobacteria, by large amount of iodide and selenium,[65] which involves these antioxidants reacting with over-reduced forms of the photosynthetic reaction centres to prevent the production of reactive oxygen species.[66][67]
Oxidative stress in disease
Further information: Pathology, Free-radical theory, and Oxidative stress
Oxidative stress is thought to contribute to the development of a wide range of diseases including Alzheimer's disease,[155][156] Parkinson's disease,[157] the pathologies caused by diabetes,[158][159] rheumatoid arthritis,[160] and neurodegeneration in motor neuron diseases.[161] In many of these cases, it is unclear if oxidants trigger the disease, or if they are produced as a secondary consequence of the disease and from general tissue damage;[52] One case in which this link is particularly well understood is the role of oxidative stress in cardiovascular disease. Here, low density lipoprotein (LDL) oxidation appears to trigger the process of atherogenesis, which results in atherosclerosis, and finally cardiovascular disease.[162][163]
Oxidative damage in DNA can cause cancer. Several antioxidant enzymes such as superoxide dismutase, catalase, glutathione peroxidase, glutathione reductase, glutathione S-transferase etc. protect DNA from oxidative stress. It has been proposed that polymorphisms in these enzymes are associated with DNA damage and subsequently the individual's risk of cancer susceptibility.[164]
A low calorie diet extends median and maximum lifespan in many animals. This effect may involve a reduction in oxidative stress.[165] While there is some evidence to support the role of oxidative stress in aging in model organisms such as Drosophila melanogaster and Caenorhabditis elegans,[166][167] the evidence in mammals is less clear.[168][169][170] Indeed, a 2009 review of experiments in mice concluded that almost all manipulations of antioxidant systems had no effect on aging.[171]